FDA Approves First-Ever Glucose Monitoring System for Weight Loss from Signos
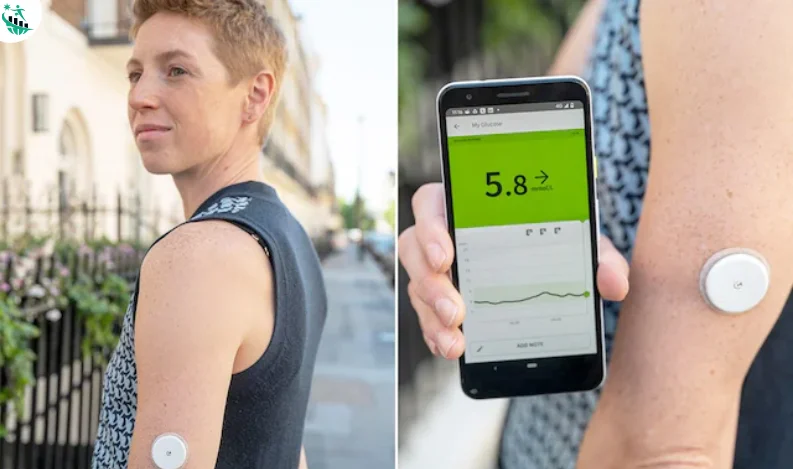
The U.S. Food and Drug Administration has approved the first glucose monitoring system designed specifically for weight loss, developed by California-based startup Signos. The approval marks a breakthrough in the nation’s fight against obesity, offering a new tool for weight management beyond drugs and surgery.
Unlike popular weight loss medications such as Novo Nordisk’s Wegovy or Eli Lilly’s Zepbound, which are typically prescribed for patients with obesity or a high body mass index (BMI), the Signos system is available to anyone seeking to manage their weight. The program combines an AI-powered platform with an off-the-shelf continuous glucose monitor (CGM) from Dexcom, providing real-time insights and lifestyle recommendations.
“There is now a solution that everybody can use to help on the weight loss journey,” said Signos co-founder and CEO Sharam Fouladgar-Mercer. “You don’t have to be a certain number of pounds to use it. We are here to help people at any point in their journey.”
The Centers for Disease Control and Prevention estimates the obesity epidemic costs the U.S. health-care system more than $170 billion annually, with nearly 74% of Americans overweight or obese. Signos hopes to address this challenge by making weight loss more accessible and affordable.
The company currently offers three- and six-month membership plans priced at $139 and $129 per month, significantly lower than the roughly $1,000 monthly cost of GLP-1 drugs. While insurers do not yet cover the system, Signos is in discussions with health insurers and employers to expand coverage.
The system can also be used alongside GLP-1 medications or bariatric surgery, or as a post-treatment option to maintain weight loss. By tracking glucose responses to food and exercise, the app aims to empower users to make informed lifestyle choices for long-term health.
Tens of thousands of patients have already tried the program, according to Signos, which has increased its inventory and software capacity to meet anticipated demand following the FDA’s approval.



Recent Comments:
No comments yet.